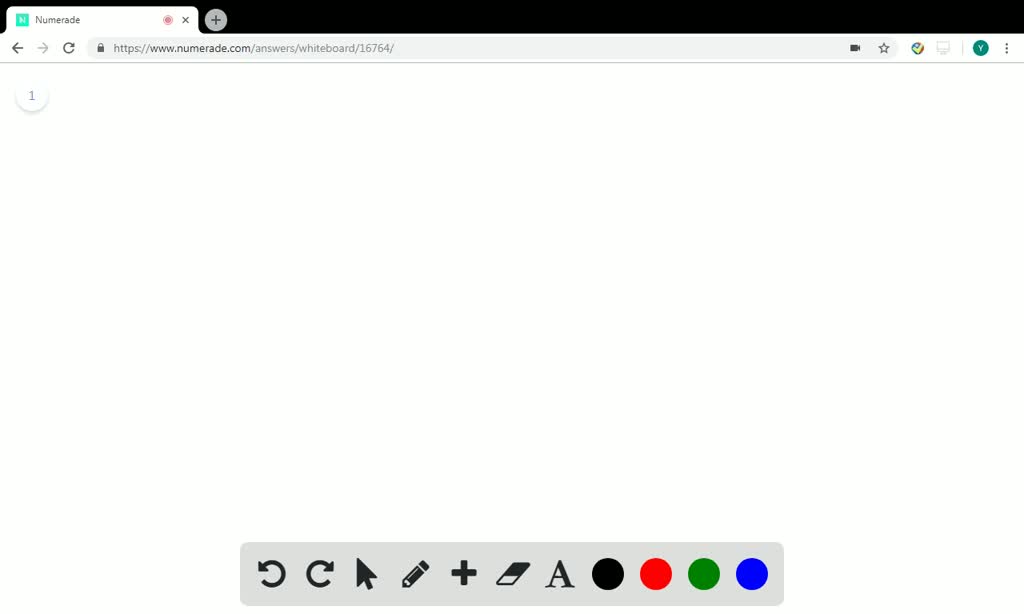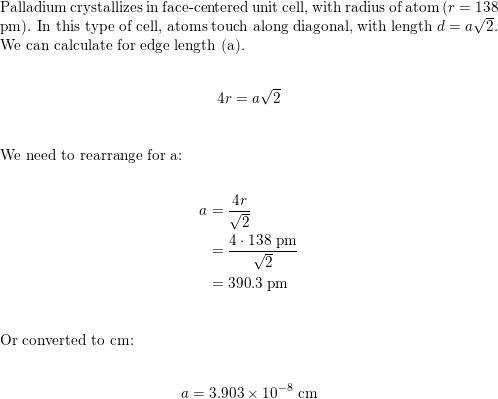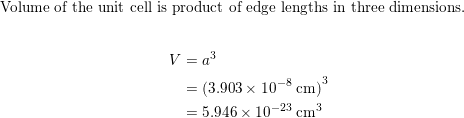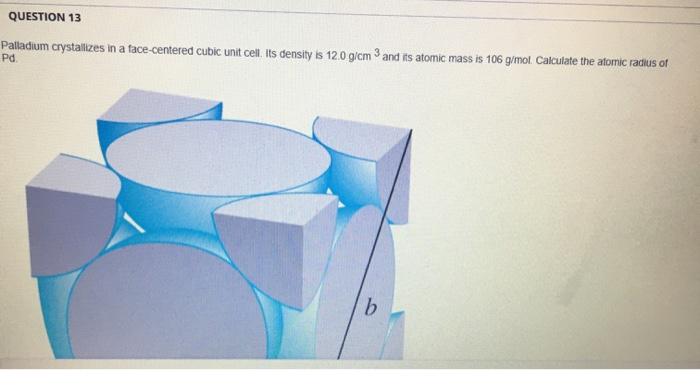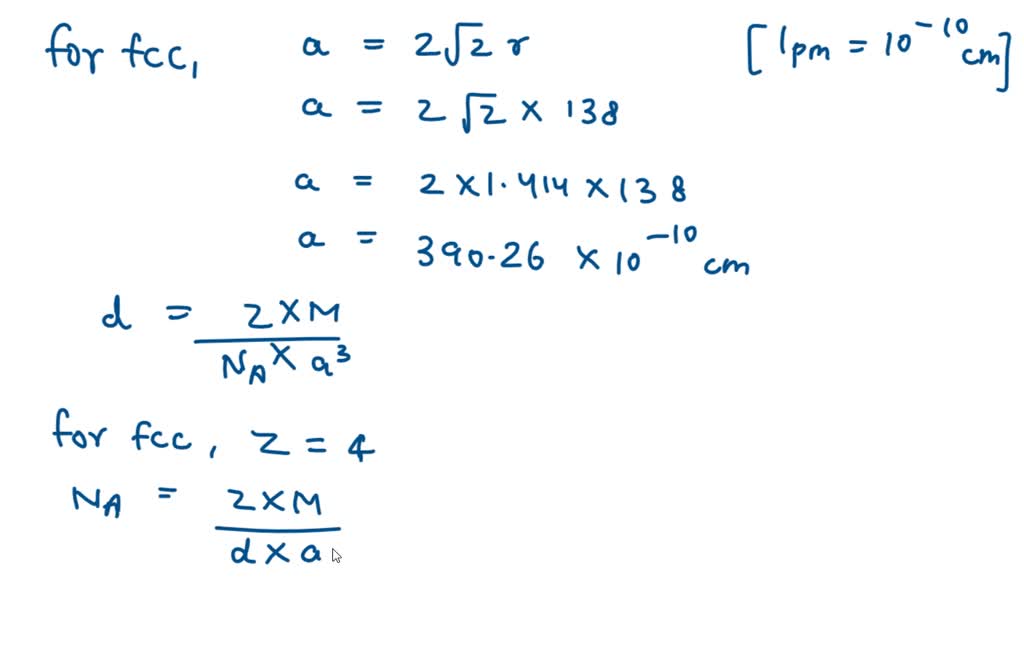
SOLVED: Palladium crystallizes with a face-centered cubic structure. It has a density of 12.0 g / cm^3, a radius of 138 pm, and a molar mass of 106.42 g / mol. Use
![SOLVED: '18. The metal palladium crystallizes in = face-centered cubic lattice with an edge length of 388.8 pm What is the density of palladium? 0.751 gcm gfem? c. 15 gcm] d. 6.01 glcmJ 412 gem?' SOLVED: '18. The metal palladium crystallizes in = face-centered cubic lattice with an edge length of 388.8 pm What is the density of palladium? 0.751 gcm gfem? c. 15 gcm] d. 6.01 glcmJ 412 gem?'](https://cdn.numerade.com/ask_previews/55b28a-c8fe-ad-ea7c-45c8c21a752_large.jpg)
SOLVED: '18. The metal palladium crystallizes in = face-centered cubic lattice with an edge length of 388.8 pm What is the density of palladium? 0.751 gcm gfem? c. 15 gcm] d. 6.01 glcmJ 412 gem?'

The metal palladium crystallizes in a face-centered cubic lattice with an edge length of 388.8 pm. What is the density of the palladium? | Homework.Study.com

Document - Problem #1: Palladium crystallizes in a face-centered cubic unit cell. Its density is 12.023 g/cm3. Calculate the atomic radius of palladium. | Course Hero

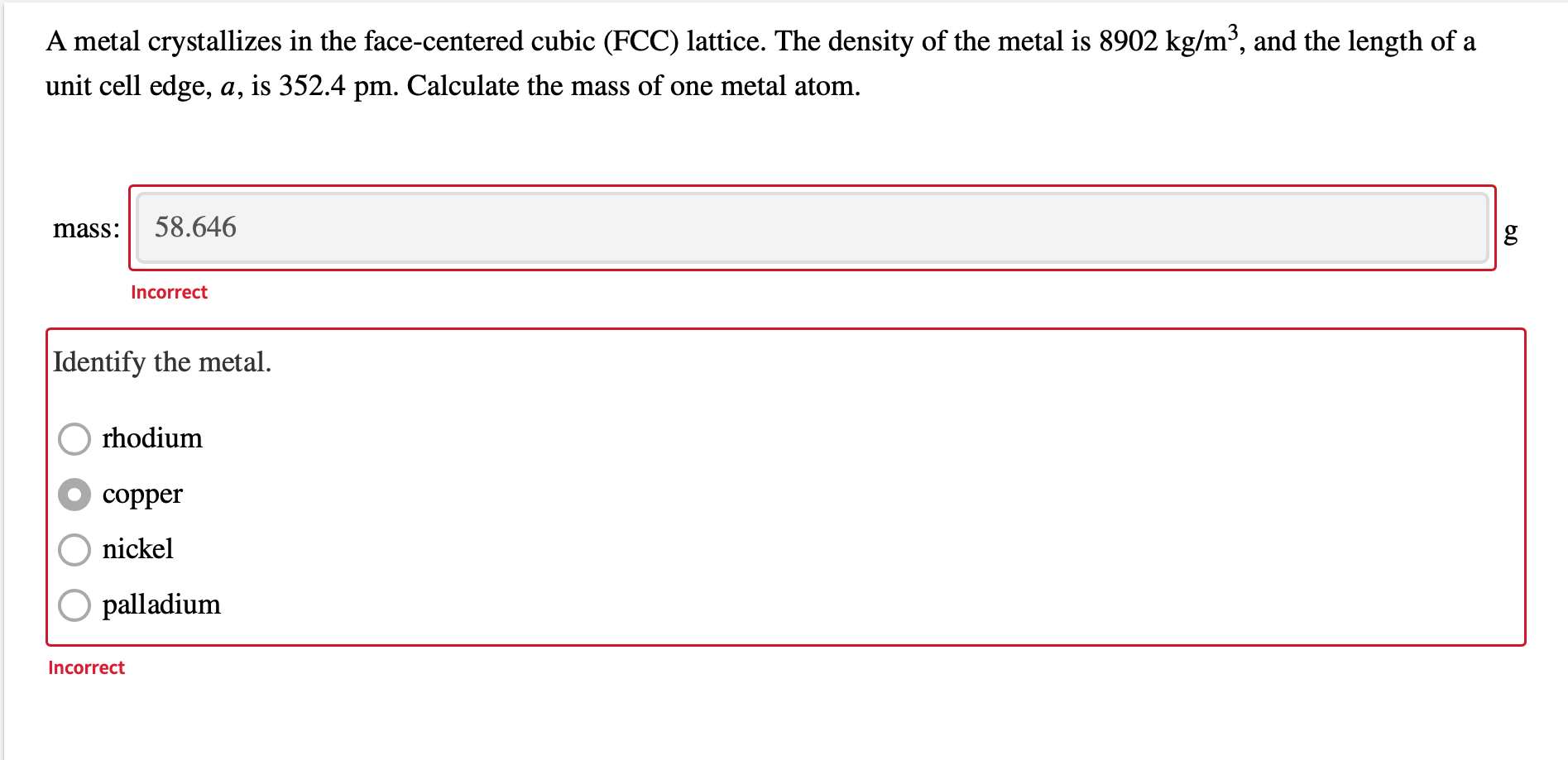
OneClass: A metal crystallizes in the face-centered cubic (FCC) lattice. The density of the metal is ...

OneClass: Palladium crystallizes with a face-centered cubic structure. It hasa density of 12.0 g/cm3,...
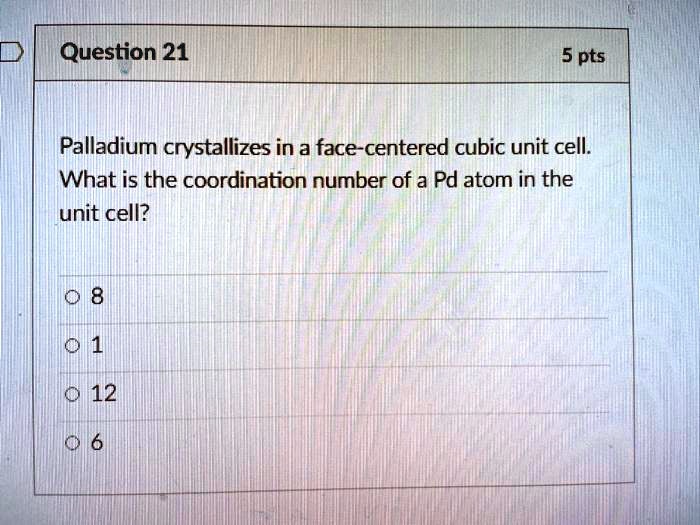
SOLVED: Question 21 5 pts Palladium crystallizes in a face-centered cubic unit cell: What is the coordination number of a Pd atom in the unit cell? 12

SOLVED: 1. Palladium (at. wt. = 106) crystallizes in a face-centered cubic unit cell. Its density is 12.023 g/cm3. Calculate the atomic radius of palladium and its packing efficiency.

SOLVED: Palladium metal crystallizes in a cubic closest packed structure. The face-centered cubic unit cell edge is 390 pm. What is the density of palladium metal? A. 13.9 g/cm³ B. 12.9 g/cm³
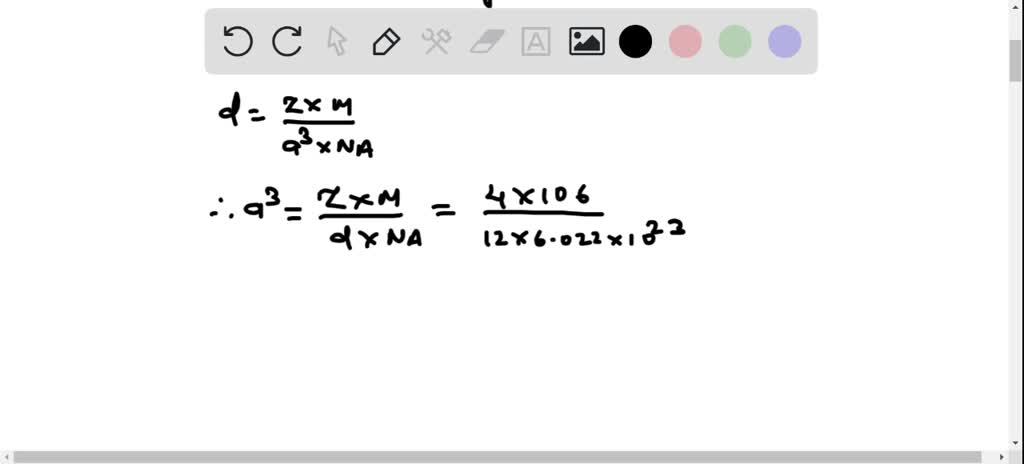
SOLVED: Palladium crystallizes in a face-centered cubic unit cell. Its density is 12.0 g/cm³. Calculate the atomic radius of palladium: (a) 138 pm, (b) 1.95 * 10⠻⠹ nm, (c) 1.95 *