FDA warns eye drops may cause infection. Here's a list of 27 products to which the alert applies. - CBS News
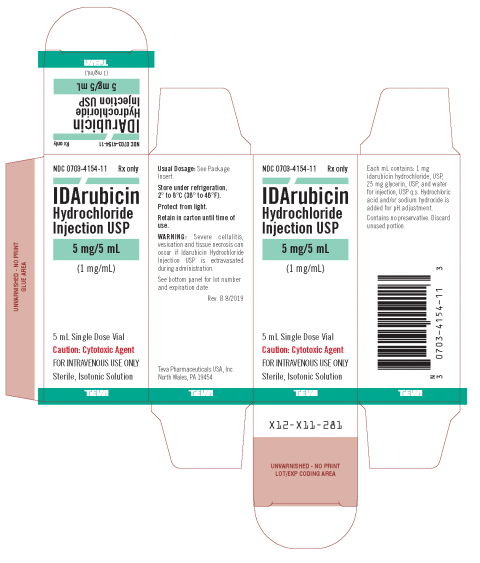
Teva Issues Voluntary Nationwide Recall of One Lot of IDArubicin Hydrochloride Injection USP 5 mg/5 mL Due to the Presence of Particulate Matter | FDA

Teva Pharmaceuticals USA, Inc., Issues Voluntary Nationwide Recall of Specific Lots of Fentanyl Buccal Tablets CII Because of Labeling Error | ONS Voice

Teva Pharmaceuticals USA, Inc. Initiates Voluntary Nationwide Recall of Metformin Hydrochloride Extended-Release Tablets USP 500 mg and 750 mg Due to Detection of N-Nitrosodimethylamine (NDMA) | FDA