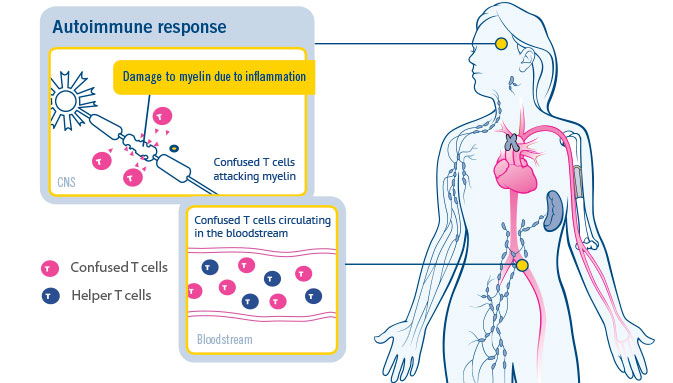
Copaxone Glatiramer Acetate injection, Treatment: Multiple Sclerosis, 1ml, Prescription at Rs 800/piece in Nagpur

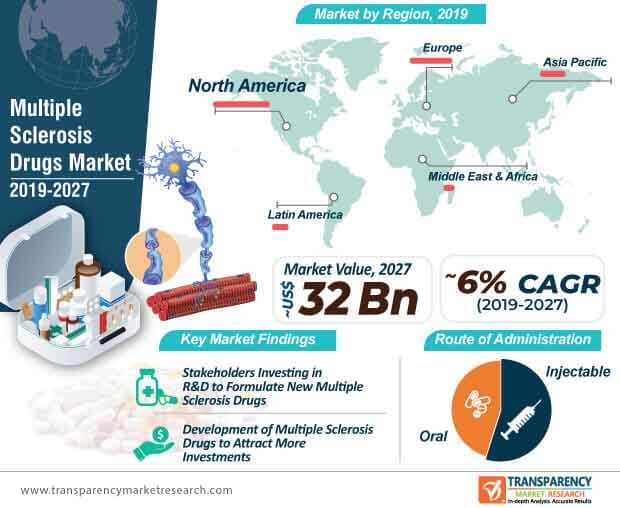
Multiple Sclerosis Drugs Market Size to Surpass USD 31.90 Billion by 2030, exhibiting a CAGR of 5.9%

Teva Kickback Trial Paused By Appeal | MedTruth - Prescription Drug & Medical Device Safety | Informed Advocacy



![PDF] Multiple sclerosis review. | Semantic Scholar PDF] Multiple sclerosis review. | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/24aa93d211cb6a26b6ecd07c8e3393b664669d1d/2-Table1-1.png)